
insights
Sprinting Towards the Finish Line – How FDA’s Breakthrough Devices Program Helps Sponsors Expedite their Development Programs
The Breakthrough Devices Program is a voluntary program for certain medical devices and device-led combination products that provides more effective access to novel treatment or diagnosis of life-threatening or irreversibly debilitating diseases or conditions. Evidence of “more effective” is assessed by the U.S. Food and Drug Administration (FDA) against current standards of care (SOC) in the U.S. As such, an applicant should demonstrate that when the device functions as intended, it is a more effective treatment for an identified disease or patient population compared with any existing treatments.
For purposes of the Breakthrough Devices Program, a condition is considered “life-threatening” if the likelihood of death is high unless the course of the disease is interrupted in a population or subpopulation. A condition or disease is considered “irreversibly debilitating” if it is associated with morbidity that has a substantial impact on day-to-day functioning and is also based on the likelihood that if left untreated, will progress to a more serious disease or condition.
The goal of the program is to provide patients and healthcare providers with timely access to these devices by speeding up their review and development while still preserving the standards for approval. FDA encourages participation in this program because there is a need to get these innovative technologies out to the public.
The number of designations has increased every year since the program launched in 2015. Early in 2022, FDA provided a sign that it could set a new record for the year. See Figure 1.
Figure 1. Number of granted Breakthrough Device Designations by fiscal year
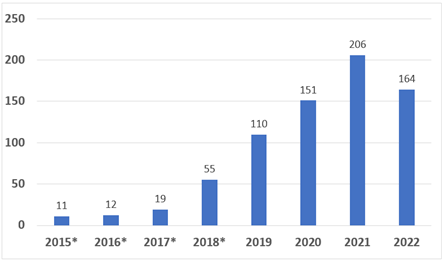
Source: https://www.fda.gov/medical-devices/how-study-and-market-your-device/breakthrough-devices-program
However, the number of devices granted authorization has remained low (56 authorizations out of a total of 728 granted designation as of October 2022). This is in part due to a large number of breakthrough applications that must be reviewed by the same Center for Devices and Radiological Health (CDRH) reviewers who review premarket notifications and other medical device submissions.
Another reason is the increased level of scrutiny on what constitutes a breakthrough device. FDA takes very seriously their assessment of innovative technologies by familiarizing themselves with similar technologies and device benefit-risk assessments. As they learn more about innovative technologies and how to better evaluate them, they provide clarity to the industry on the considerations.
One recent piece of clarity came when a draft guidance by the FDA was released for public comment in October 2022 on “Select Updates for the Breakthrough Devices Program Guidance: Reducing Disparities in Health and Health Care.” This guidance further clarifies how the Breakthrough Devices Program could benefit groups affected by health inequities and disparities. Additionally, FDA proposes to add language regarding the level of evidence needed to demonstrate an innovative technology as “more effective” that would vary according to intended use, its technology, and current standard of care. All this activity has us anticipating many more Breakthrough Devices Program applications to be submitted.
While device manufacturers work through the draft guidance and prepare feedback and comments to FDA – if they wish to do so – we also recommend they fully understand the program’s benefits, challenges, and submission requirements to fully prepare for the program if this aligns with their strategy.
In this article, we will seek to break down the program’s components and provide some recommendations when deciding if applying for the program is a fit for your organization.
Program Eligibility and Benefits
For consideration into the program, manufacturers must apply through a Q-submission that details the breakthrough technology and why it’s a more effective treatment or diagnosis for a debilitating or life-threatening disease than any existing alternative treatments. Q-submissions (or Q-subs for short) are feedback mechanisms used by submitters to request FDA feedback about medical device submissions. These feedback mechanisms could be in the form of a written response or a meeting depending on the request that is made by the submitter. Examples of Q-submissions include the following:
- Pre-Submissions: a formal written request is made by the submitter to FDA on topics related to an intended premarket submission such as cybersecurity, performance testing, non-clinical or clinical study protocols
- Informational Meetings: a request to share information with FDA to help familiarize them with a new device or technology without the expectation of feedback
- Study Risk Determinations: a request for FDA determines whether a planned clinical study is considered a significant risk, non-significant risk, or exempt from Investigational Device Exempt (IDE) regulations
Criteria for selection into the Breakthrough Devices Program are based on Section 515(b) of the Federal Food, Drug, and Cosmetic Act where the candidate device is subject to a future marketing authorization through the PMA, 510(k), or De Novo pathways. The designation criteria for devices are as follows:
- Provide for more effective treatment or diagnosis of life-threatening or irreversibly debilitating human disease or conditions
- Represent breakthrough technologies
- Offer significant advantages over existing approved or cleared alternatives
FDA will review the application and provide a decision to grant or deny the request for designation within 60 calendar days. More information on device eligibility for the program can be found here.
If a manufacturer’s program is chosen as a breakthrough device, their development program will have the advantage of frequent interactions with FDA to gather expedited feedback. Often key FDA reviewers and senior management are involved in these feedback discussions, so it’s an opportunity for the manufacturer to gain guidance on what is required for approval and how to meet those requirements. The Breakthrough Devices Program offers an opportunity for the manufacturer to engage with FDA early and often to align on clinical protocol development. Besides clinical study development, other activities that can be reviewed interactively with FDA as part of the program include medical device (including Software as a Medical Device (SaMD)) development activities including pre-clinical studies and appropriate regulatory pathway determination.
Points to Consider Prior to Application and Strategy Recommendations
If you’re thinking about getting your innovative device to market in a timelier manner, the Breakthrough Devices Program may be appropriate for your technology. Before applying, consider the following impacts on your internal timelines. Although the program is intended to expedite development, it’s important to ensure your organization is prepared to embark on this exciting journey with FDA. With expedited feedback and priority review comes an impact on development timelines and organizational priorities.
Here are a few ways you can prepare even before submitting your application to the program:
- Confirm your organization, including senior management, are on board for embarking on this effort
- The organization’s regulatory team should get up to speed on the considerations and requirements of the Breakthrough Devices Program. They should inform their leadership about the criteria for eligibility and discuss it as a potential option. Ensure the entire organization understands the process for application and what acquiring designation means not only for regulatory approval but also for the organization and development timelines.
- Consider how receiving the breakthrough designation will impact development timelines
- Anticipate if there are dependencies amongst the various development activities, if these activities can and should be communicated with FDA via sprint discussions, and consider the need for contingency plans in case there are delays. There is a good chance the device under breakthrough designation may need clinical data to support its safety and effectiveness. Other development activities that could impact timelines should also be considered; these include a design freeze, verification, and validation of the completed device.
- Identify team roles and responsibilities, including preparing for and submitting the application, and strategies for managing success
- Work internally to determine the specific topics your team wishes to discuss with FDA, anticipated sprint cadences based on internal sprint durations, and the definition of ‘done’ required to reach alignment with FDA. Propose interaction frequency with FDA and provide potential supporting design and other documentation for early FDA review.
Conclusion
The Breakthrough Devices Program is a great opportunity for device manufacturers who have determined they have a novel technology that can serve an underserved population or can dramatically improve clinical outcomes. To that end, a manufacturer should ensure they’ve thoroughly reviewed the current landscape including alternative or similar devices or treatments, and compare and contrast with their proposed device. Even if an application is not granted breakthrough designation, this review will still be invaluable as a manufacturer demonstrates the device’s benefits and risks in all premarket submissions.
Manufacturers considering seeking this designation shall also consider the impact on their organization in terms of shifts to planning, development, and design transfer timelines. Regulatory teams should work closely with leadership and their development teams to plan the breakthrough application and the process for each workstream discipline if the designation is granted.
The Breakthrough Devices Program is a major milestone for companies of all sizes. A breakthrough designation can help manufacturers expedite the marketing of important innovative devices through early and frequent interactions with FDA. Inclusion into the program may also help manufacturers with setting device standards for other manufacturers within the device space or improved investor relations and fundraising.
Halloran will be releasing more content on FDA programs similar to the Breakthrough Devices Program. Meanwhile, if you’re considering applying to the program, please contact a member of our team today to discuss regulatory strategies.




