insights
Reimagining Clinical Studies to Support Accelerated Approval for Anticancer Products
There is, and always has been, a need for speed in drug development. While, in many ways, speed favors both profits and patients, there is a tension between the race to market and the need for thorough clinical evaluation. This tension puts global health authorities, like the U.S. Food and Drug Administration (FDA), in a challenging position because it requires they effectively safeguard the public against unsafe or ineffective drugs without unduly delaying the availability of much-needed new therapies.
Historically, oncology drug development has operated in the fast lane, driven by a benefit-risk profile that favors speedy delivery of promising new therapies to patients with poor prognoses and limited therapeutic options. However, recent developments have signaled a paradigm shift in oncology-directed drug development, particularly as it relates to molecularly targeted therapies. As increasingly safe and efficacious targeted therapies emerge, the benefit-risk landscape for approval of new oncology drugs continues to evolve.
A major recent inflection point in this evolution was the FDA’s launch of Project Optimus and the corresponding efforts to improve safety and tolerability by optimizing dose selection. More recently, FDA has turned its focus to optimizing the Accelerated Approval (AA) pathway for anticancer products.
The AA pathway was launched in the early 1990s, largely in response to the HIV/AIDS epidemic after advocates urged the FDA to streamline efficacy requirements to expedite access to innovative products that show promise for serious, incurable diseases. In the subsequent decades, the AA pathway has allowed FDA to approve drugs that address unmet medical needs and to put promising new therapies in the hands of underserved patient populations more quickly than the traditional regulatory approval processes would allow. Through the AA pathway, sponsors can seek conditional product approval based on limited clinical data using a surrogate or intermediate endpoints. Full product approval is then typically obtained by way of a confirmatory clinical study using more traditional clinical endpoints. Importantly, the number of drugs approved under the AA pathway has increased significantly over the last several years, from less than 10 in 2010 to over 40 in 2020,1 and the FDA has been criticized regarding recent controversial approvals using AA, such as aducanumab.
The FDA’s Office of Oncologic Drugs (OOD) and the Oncology Center of Excellence (OCE) have taken note of the existing concerns associated with the AA pathway. Through multiple Oncologic Drugs Advisory Committees (ODACs) facilitated in 2021 and 2022, the FDA has worked to revisit and reconsider the existing approvals for several products approved under AA. The effort was primarily intended to scrutinize the confirmatory trials for these products to determine if they had been appropriately designed, and executed, and provided available data to support a traditional approval. As an outcome of these ODACs, multiple approvals were withdrawn.
Of the products evaluated in these ODAC meetings, several maintained approval years after confirmatory studies failed to demonstrate clinical benefit. In the review of such data, FDA highlighted a clear need for more frequent surveillance and assessment(s) of existing AA approvals, and perhaps a reimagining of the clinical studies (and corresponding data) required to support AA for anticancer products.
New Draft Guidance
In March 2023, as a next step toward reimagining AA for oncology products, the FDA released a draft guidance titled “Considerations to Support Accelerated Approval of Oncology Therapeutics.” As a central focus of this new guidance, the FDA takes aim at improving upon challenges relating to the use of single-arm studies for AA and the corresponding design and timing of confirmatory trials.
While a variety of trial designs have historically been used to support AA, single-arm trial designs have most commonly been used in oncology. Single-arm studies allow drug developers to expedite approval, particularly for small sub-populations of treatment-refractory patients that may present ethical challenges to enrolling in a large, randomized study. Still, there are limitations and concerns to the use of single-arm trials in support of AA and these limitations can introduce significant uncertainty pertaining to safety and efficacy assessments.
While single-arm studies may remain an allowable option in select circumstances, the guidance illustrates FDA’s preference for AA based on randomized data. Notably, the guidance describes a potential advantage of randomized controlled trials (RCTs) when compared to a single-arm trial, sharing that use of the one-trial approach, in some cases, may not require separate clinical trials because longer-term follow-up in the same trial could fulfill a post-marketing requirement to verify clinical benefit.
In addition to the proposed alternatives to single-arm studies, the guidance highlights challenges associated with design, implementation, and oversight of confirmatory studies that must be performed to convert a conditional (accelerated) approval to full approval. Until recently, there was little enforcement related to the timing of these confirmatory studies which led to “dangling approvals” or otherwise large gaps between the accelerated approval and the availability of confirmatory data to support the long-term durability of the therapeutic response. FDA expects to close the gap between confirmatory trials and AA requiring that confirmatory studies are well underway, though fully enrolled would be ideal, at the time AA is granted.
For sponsors, there is much to consider as they evaluate the strategic and financial impact(s) of the FDA’s emerging recommendations relating to AA in oncology. In an effort to illustrate the potential impact of FDA’s recommendations, we will briefly examine the clinical development plan leading to AA for Lytgobi® (futibatinib) and provide a hypothetical example of how this particular clinical development strategy may change in the context of this guidance’s model. The comparative timeline will be critical for sponsors to understand as they navigate their roadmap before applying for AA for their oncology product so they can plan accordingly.
Futibatinib Overview
“Futibatinib (TAS-120) is an oral, potent, selective tyrosine kinase inhibitor of FGFR1, 2, 3 and 4. Futibatinib selectively binds to the ATP binding pocket of FGFR1-4 resulting in the inhibition of FGFR-mediated signal transduction pathways, reduced tumor cell proliferation, and increased tumor cell death in tumors with FGFR1-4 genetic aberrations.”2
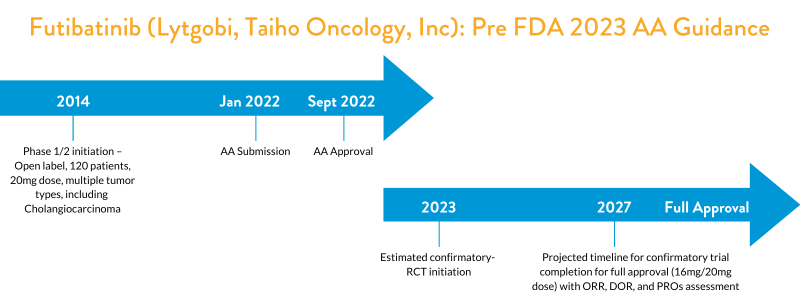
Futibatinib Clinical Development and Accelerated Approval (Prior to FDA’s 2023 Draft Guidance)
FDA granted AA to futibatinib in September 2022 for adult patients with previously treated, unresectable, locally advanced or metastatic intrahepatic cholangiocarcinoma harboring fibroblast growth factor receptor 2 (FGFR2) gene fusions or other rearrangements. “Efficacy was evaluated in TAS-120-101 (NCT02052778), a multicenter, open-label, single-arm trial that enrolled 103 patients with previously treated, unresectable, locally advanced, or metastatic intrahepatic cholangiocarcinoma harboring an FGFR2 gene fusion or other rearrangement.”3 The major efficacy outcome measures were overall response rate (ORR) and duration of response (DOR) as determined by an independent review committee according to RECIST v1.1 (Response Evaluation Criteria in Solid Tumors). The recommended futibatinib dose is 20 mg orally once daily until disease progression or unacceptable toxicity occurs. This dose was likely based on the maximum tolerated dose (MTD) from the Phase 1 dose escalation studies.
As a condition for the AA, FDA asked the sponsor to conduct an RCT comparing futibatinib at two dose levels (16 mg and 20 mg once daily) to verify the clinical benefit of futibatinib in patients with advanced or metastatic cholangiocarcinoma harboring FGFR2 gene fusion or other rearrangements. This confirmatory study has a projected trial completion timeline of 2027 (NCT05727176). FDA expects the study to enroll a minimum of 120 patients, with an assessment of ORR and DOR by a blinded independent review and evaluation of additional endpoints such as Patient Reported Outcomes (PRO). FDA also expects this trial to have an adequate representation of racial and ethnic minorities in the trial population to mirror real-world patients.
FDA’s recommendation for the inclusion of two dose levels (16mg/20mg) within the confirmatory study reflects recent initiatives from the OCE, Project Optimus, and the January 2023 guidance on dose optimization considerations for developers of precision oncology therapies. Ultimately, FDA is urging oncology drug developers to perform more thorough dose optimization to avoid the long-term commercial use of suboptimal doses. With a new draft guidance on this topic now released, sponsors are encouraged to incorporate dose optimization into their investigational clinical development plan to gain early alignment with FDA on the intended and optimal commercial dose level.
ClinicalTrials.gov Identifier: NCT02052778
In futibatinib’s example, the timeline for the completion of Phase 1 b trial in refractory patients is longer than the average single-arm trials conducted by other sponsors. The inclusion of multiple tumor types in this study (NCT02052778) could be one reason for the long duration.
Post–FDA’s 2023 Guidance on Clinical Considerations for Accelerated Approval
With the issuance of the March 2023 FDA guidance on “Considerations to Support Accelerated Approval of Oncology Therapeutics,” sponsors targeting to initiate a clinical trial in 2023 for a subsequent AA may now consider the option of conducting a single RCT which includes both AA endpoints (Overall response rate (ORR), Progression Free Survival (PFS), or alternate endpoints) and long-term endpoints such as Overall Survival (OS), Duration of Response (DOR), and PROs to enable a seamless transition from AA to full approval.
Early discussions with FDA based on preliminary clinical data and a proposed clinical study plan is highly recommended before initiating a single study approach. Careful consideration of comparator choice is also critical to facilitate the regulatory decision upon completion of the study, given the evolving Standard of Care (SOC).
A sponsor who initiated a single RCT for AA in 2014 is likely to achieve full approval well ahead of what would be expected under the old paradigm (i.e., a single-arm study followed by RCT for confirmatory study initiated after AA). Moreover, the timing to AA and full approval in the example below may likely be further accelerated depending on the nature of the study.
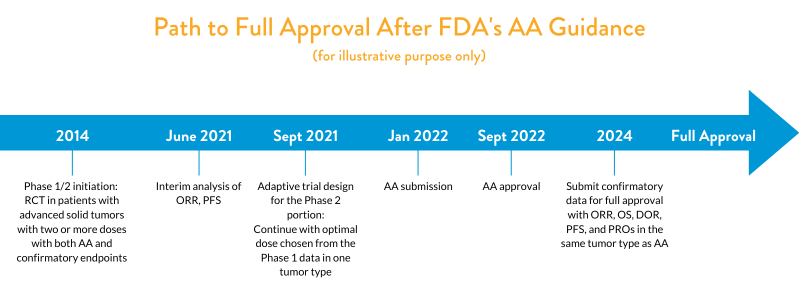
As illustrated in the above figure, a sponsor initiating a single RCT in multiple advanced solid tumor types in refractory patients should plan on incorporating at least two or more doses in the Phase 1/2 studies, chosen based on initial dose-ranging studies. After assessing early clinical data, sponsors may explore an adaptive trial incorporating both pre-specified AA endpoints (e.g., ORR, PFS) and confirmatory study endpoints (e.g. OS, DOR, clinical outcomes such as PROs) in Phase 1/2. After an interim analysis of ORR and PFS data, they may choose to collect more data from one tumor type if they see early efficacy and a good safety profile compared to the SOC. Once sufficient data is obtained, as pre-specified in the protocol, they could apply for AA in that tumor type. Following AA, sponsors can then follow up with a full confirmatory data package now strengthened with OS, DOR, and PROs data, collected over a longer duration.
This “one-trial” approach (as outlined in the futibatinib example) should include multi-arm studies with interim analyses for futility or use other adaptive trial design elements such as sample size re-estimation. FDA advises in its March 2023 guidance that “analyses of efficacy to support accelerated approval should be avoided until the trial is close to or fully enrolled to mitigate potential challenges in accrual if an accelerated approval is granted.” The sponsor must also ensure they are maintaining the study blindly and picking both short- and long-term endpoints that are robust and clinically meaningful.
As an alternative approach, the guidance offers a CDP containing two randomized controlled clinical trials (one for AA and one for full approval). For this approach, FDA mentions in its 2023 guidance the sponsor may want to study the same cancer type but in another line of therapy, especially an earlier disease setting. This allows the sponsor to expand its indication when the second trial reads out and full approval is granted and reflects another OCE initiative, such as “Project Frontrunner.”
Lastly, while the option of conducting a single-arm study for AA is also available to sponsors on a case-by-case basis, as was used for futibatinib, we anticipate FDA will exhibit an increasing preference for the RCT-based designs discussed above. Importantly, sponsors pursuing this approach must now ensure that patient enrollment for the confirmatory study is well underway at the time of AA. While this requirement presents clear financial challenges, particularly for smaller organizations progressing with their first asset, it aims to decrease the post-marketing time spent with residual uncertainties regarding the relationship between the surrogate or intermediate endpoint to the ultimate clinical benefit. Moreover, it may reduce additional challenges with payors looking for full approval based on efficacy and safety for reimbursements.
Conclusion
FDA’s recent guidance, “Considerations to Support Accelerated Approval of Oncology Therapeutics,” is the most recent inflection point in FDA’s increasingly visible initiative to revisit and optimize the AA process. The recommendations included in the guidance take strides toward a future paradigm where investigational products are more thoroughly vetted in an expedited manner. Ultimately, this paradigm shift offers an opportunity to improve selectivity in the overall approval process and safeguard patients against long-term commercial availability of products that do not provide a clear clinical benefit while also exhibiting a favorable safety profile.
Still, there is more room for the FDA and industry to work together in the ongoing improvement of the AA process. In addition to revised clinical designs and improved monitoring of confirmatory studies, the FDA could consider providing expanded guidance relating to the development and validation of biomarkers (for use as surrogate endpoints), particularly in understudied cancer subtypes. Reducing subjectivity relating to the acceptance of unvalidated surrogate endpoints and the corresponding assessment of data required to support a “substantial improvement” over available therapies may serve to minimize severely complicating disagreements during the final review processes.
Given how active the OCE has been in the last few years, Halloran certainly expects to see more guidance and future initiatives that enable us to rethink oncology drug development. In the meantime, we are continually working with sponsors to navigate emerging changes in the field to effectively collaborate with FDA in the development of robust clinical development and regulatory strategies.
Contact us today to discuss your regulatory strategy, health authority meeting preparation, and clinical trial design for your oncology drug development. We’re ready when you are.
References:
1. Batta A, Kalra BS, Khirasaria R. Trends in FDA Drug Approvals Over the Last 2 Decades: An Observational Study. J Family Med Prim Care. 2020;9(1):105-114
2. Taiho Pharma Press Release. U.S. FDA Approves FGFR Inhibitor, Futibatinib for the Treatment of Previously Treated, Unresectable, Locally Advanced or Metastatic Intrahepatic Cholangiocarcinoma. Dated 3 October 2022. https://www.taiho.co.jp/en/release/2022/20221003.html
3. FDA. FDA grants accelerated approval to futibatinib for cholangiocarcinoma. Current as of 30 September 2022. https://www.fda.gov/drugs/resources-information-approved-drugs/fda-grants-accelerated-approval-futibatinib-cholangiocarcinoma